Iran Sheikhshoaie* and Ahmad Kamali
Department of Chemistry, Shahid-Bahonar Universirt of Kerman, 76175-133 Kerman (Iran)
Article Publishing History
Article Received on :
Article Accepted on :
Article Published :
Article Metrics
ABSTRACT:
Disulphide derivatives of imine compounds have the subject of intensive investigation over the past years, reflecting that they are excellent ligands for coordination to the metal ions. The disulphide group is well recognized as a potential binding site for metal ions in biological systems. In our present works we prepared two symmetrical Schiff base compounds N,N/-bis (2-hydroxy-3-methoxybenzylidene)-2,2/-(aminophenylthio) ethane [L1] and N,N/-bis (2-hydroxybenzylidene)-2,2/-(aminophenylthio) ethane [L2] by condensation of bis-(2-aminophenyl) disulphide with salen derivatives in absolute ethanol at room temperature. The structures of prepared compounds were checked by several spectroscopic methods. In the other part of this work the structure of these compounds have been optimized in gas phase and all quantum chemical parameters of the optimized L1 and L2 compounds have been discussed by some semi-empirical methods.
KEYWORDS:
Sciff base; quantum parameter; bis (2- aminophenyl) disulphide; semi-emperical method; corrosion inhibition
Copy the following to cite this article:
Sheikhshoaie I, Kamali A. On the structural property of two symmetrical N2O2-coordination Schiff base compounds. Orient. J. Comp. Sci. and Technol;1(1)
|
Copy the following to cite this URL:
Sheikhshoaie I, Kamali A. On the structural property of two symmetrical N2O2-coordination Schiff base compounds. Orient. J. Comp. Sci. and Technol;1(1). Available from: http://www.computerscijournal.org/?p=2013
|
Introduction
There has been considerable activity concerning the synthesis of Schiff base ligands. These compounds have significant importance in chemistry; especially in the development of Schiff base complexes, because Schiff base ligands are potentially capable of forming stable complexes with
metal ions1-6. Some imines or Schiff base compounds and their metal complexes are also noted for their significant antimicrobial activities7, 8 and corrosion inhibition9. Some organic compounds, which adsorb on the metal surface and suppress metal dissolution and reduction reaction are named
inhibitors or anti corrosion materials10. In many cases, the role of inhibitors is to from a surface coating one or several molecular layers thick that serves as a barrier11. Chemisorption is probably the most important type of interaction between the metal surface and an inhibitor molecule12.
In the other hand the structure and confrontations of L1 and L2 plays an important role in the structural properties. In the present study the correlation between the molecular structures and the coordination sites of L1 and L2 ligands have been investigated by using some semi-imperial quantum chemical calculations.
Recently, we have reported some new schiff base ligands13,14 and in this article, we will report synthesis and the structural properties of L1
and L2 schiff base compounds[Scheme1] by the reaction of 1,2-di (ortho-aminophenylthio)ethane with 3-methoxysalicylaldehyde or salicylaldehyde.
Experimental
Instrumental and methods
Elemental analyses were performed on a Perkin Elmer analyzer, 1H-NMR and 13C-NMR
![Scheme 1: The Structure of N,N/-bis (2-hydroxy-3-methoxybenzylidene)-2,2/-(aminophenylthio) ethane [L1] and N,N/-bis (2-hydroxybenzylidene)-2,2/-(aminophenylthio) ethane [L2]](http://www.computerscijournal.org/wp-content/uploads/2008/08/Vol01_No1_Eva_B.A_Sch1-150x150.jpg) |
Scheme 1: The Structure of N,N/-bis (2-hydroxy-3-methoxybenzylidene)-2,2/-(aminophenylthio)
ethane [L1] and N,N/-bis (2-hydroxybenzylidene)-2,2/-(aminophenylthio) ethane [L2]
Click here to View scheme
|
spectra were prepared on a Bruker AM 400 MHz, and UV-Vis spectra were reported with a Beckman DU-7000 spectrometer. FT-IR spectra were reported on a Shimadzu DR-8001. All calculation was done by a Pentium IV Personal Computer. C, H and N elemental analysis were performed on a Perkin Elmer analyzer.
Material
Salycyladehyde, 3-methylsalicylaldehyde chloroform (reagent grade Merck) was purified and dried before use according to the standard methods.
Synthesis
L1 and L2 schiff base compounds [Scheme1] by the reaction of 1,2-di (orthoaminophenylthio) ethane (0.276g, 0.001mol) with 3- methoxysalicylaldehyde (0.304 g, 0.002 mol) or salicylaldehyde (0.244g, 0.002mol) in chloroform (25mL) in the presence of one drop conc. H2SO4 under stirring at room temperature (6 h). The yellow solid compounds were recovered by filtration, washed with cold ethanol and dried over fused CaCl2. The colored imines were purified by recrytallization from ethanol (yields 55-70 %). The C, H and N analytical results and some physical properties of the isolated L1 and L2 Schiff base ligands have been shown in Table 1.
Spectroscopic data
Spectroscopic data for L1 Schiff base ligand
FT- IR νmax (KBr, cm-1): 3500 (m, OH), 3042 (w, aromatic C-H), 1641 (s, C=N), 1184 (phenolic C-O). 1H-NMR (Bruker AM 400 MHz, CDCl3 solvent with TMS as an internal standard), δ3.2 (s, 6H, CH3), 6-8.8 (d, 14H, phenyl), 10.2 (s, 2H, OH), 7.8- 8 (d, 2H, CH= N, 3.1 (4H, s, CH2). Electronic spectra: (DMF as a solvent) λmax=286 and 353 nm.
Spectrocopic data for L2 ligand
FT-IR νmax (KBr, cm-1): 1597(m, OH), 3052 (w, aromatic C-H), 1649 (s, C=N), 1192 (phenolic C-O). 1H-NMR (Bruker AM 400 MHz, CDCl3 solvent with TMS as an internal standard), 6.9-8.6 (s, 16H, Phenyl), 7.25-8.32 (s, 2H, CH= N), 13.2 (s, 2H, OH), 3.3 (s, 4H, CH2). Electronic spectra: (DMF as a solvent) λmax=278 and 349 nm.
Theoretical section
We have carried out quantum mechanical computations for L1 and L2 Schiff base ligands. Two structures were pre-optimized by the Molecular
Mechanics method and then fully optimized at the AM115 semi empirical-self-consistent field-molecular orbital (SCF-MO) method by using MOPACK 7.0 program package16. Also the theoretical electronic spectra were obtained for L1 and L2 Schiff base ligands. Theoretical calculations were performed
with MOPACK 7.0 and program and geometry optimizations were used to find the coordinates of molecular structures that represent a potential energy minimum. The lowest energy, dipole moments and energy are calculated at AM1 semiempirical method. The values of dipole moments, energies and heat of formations for L1 and L2 molecules are listed in Table 2. In Table 3, the atomic charges of S, N (in C=N group) and O (in OH group) atoms of the molecules are given. Some important theoretical electronic spectral data have been shown in Table 4.
Results and Discussion
The reaction of 1,2-di (ortho-aminophe nylthio) ethane with 3-methoxysalicylaldehyde or salicylaldehyde in chloroform in the presence of one drop conc. H2SO4 under stirring at room temperature (6 h) afforded two Schiff base ligands, N,N/-bis (2- hy d r ox y – 3 – m e t h ox y b e n z y l i d e n e ) – 2 , 2 / –
(aminophenylthio) ethane [L1] and N,N/-bis (2- hydroxybenzylidene)-2,2/-(aminophenylthio) ethane [L2]. Optimized molecular structure parameters of
the L1 and L2 molecules are summarized in Table 1 and Table 2. The coordination sites (N and O) atoms exhibit a substantial negative charge, S atoms in the two structures have positive charges and they have not any role in coordination properties of these imines. The OCH3 group is a donor electron group and plays a major role in designing of N and O coordination sites of L1 imine. The negative charge on the coordination sites for L1 (N2, N2 /, O3 and O3 /) are bigger than the negative charge on the coordination sites for L2 (see Table 2). Table 3 is shown the theoretical electronic bands for L1 and
L2 molecules, the lowest energy bands of the electronic spectra for these compounds (344.3 and 341.9 nm) are attributed to the HOMO→LUMO transitions ( 97→98 for L1 and 85→86 for L2) and this bands are related to π→π* transitions. The highest energy bands of the calculated electronic spectra (252.7 and 226.4 nm) are attributed to 97→99 electronic transition for L1 and 83→87 for L2, and related to π→π* transitions.
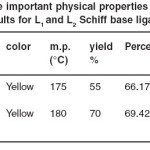 |
Table 1: Some important physical properties and analytical results for L1 and L2 Schiff base ligands
Click here to View table
|
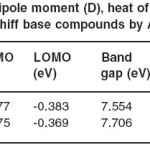 |
Table 2: Calculated values of dipole moment (D), heat of formation (kcal/mol) and energy (kcal/mol) for L1 and L2 Schiff base compounds by AM1 semi-empirical method
Click here to View table
|
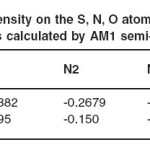 |
Table 3: Charges density on the S, N, O atoms of L1 and L2 Schiff base compounds calculated by AM1 semi-empirical method
Click here to View table
|
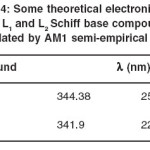 |
Table 4: Some theoretical electronic bands of L1 and L2 Schiff base compounds calculated by AM1 semi-empirical method
Click here to View table
|
On the other hand, we can study the ability of quantum chemistry and select corrosion inhibitors. Because when two molecular systems
react, the energy variation along the reaction coordinate is due to the geometric distortion of the reagent molecules and to the interaction energy
between them. The interaction of molecular orbitals results in a decrease of the energy of the lower orbital and an increase of the energy of the higher orbital. The extent of energy change is inversely proportional to the difference in energy of the MO prior to interaction and is directly proportional to
the products of the MO coefficients at one site of interaction. According to Fukui’s frontier orbital approximate, interactions between frontier MO only,
highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of both reactants are frequently considered, since the
inverse dependence of stabilization energy on orbital energy difference ensures that terms involving the frontier MO will be larger than others (Table 3).
So L1 is a good corrosion inhibitor.
References
- V. M. Leovac, V. S. Jevtovic, L. S. Jovanovic and G. A. Bogdanovic, J. Serb. Chem. Soc 70: 393-423 (2005).
- X. Li, M. Bera, G. T. Musie, D. R. Powell, Inorganica Chimica Acta, 361: 1965-1972 (2008).
- G. Brewer, C. Brewer, R. J. Butcher, E. E. Carpenter, Inorg. Chim. Acta, 359: 1263-1268 (2006).
- M. Andrade, C. Sousa, J. E. Borges, C. Freire, J. Phys. Org. Chem. 18: 935-940 (2006).
- A. Pue, Croatica Chemica Acta, 75: 165-173 (2002).
- A. K. Singh, S. Kumari, K. Ravi Kumar, B. Sridhar, T.R. Rao, Polyhedron, 27, 1937-1941 (2008).
- M. Shakir, Y. A. H. T. N. Chishti, S. Parveen, Spectrochimica Acta Part A, 65: 490-496 (2006).
- K. Z. Imail, A. E. Dissouky, A. Z. Shehada, Polyhedron, 16: 2909 (1997).
- a.M. N. Desai, M. B. Desai, C. B. Shah, S. M. Desai, Corrosion Sci., 1986, 26, 827-837. b. H. Shoky, M. Yuasa, I. Sekin, R. M. Issa, H. Y. EI-Baradie and G. K. Gomma, Corrosion Sci., 40: 2173-2186 (1998).
- H. Derya Leçe, C. Kaan Emregül, G. Orhan Atakol, M. Mars, Corrosion Science, Volume, 50: 1460-1468 (2008).
- H. Ju, Z. Kai, Y. Li, Corrosion Science, 50: 865-871 (2008).
- M. Elayyachy, B. Hamouti, A. El Idrissi, Appl. Surf. Sci., 249: 176-182 (2005).
- I. Sheikhshoaie, M. A. Sharif, Acta Cryst., E62: o3563-03565 (2006).
- I. Sheikhshoaie, Russ. J. Coord. Chem., 3: 388-391 (2007).
- M. J. S. Dewar, E. G. Zoebisch, E. F. Healy, j. J. P. Stewart, J. Am. Chem. Soc., 107: 3902 (1985).
- J. J. P. Stewart, MOPACK, A Semiempirical Molecular Orbital Program, QCPE, 455: 6 (1983).

This work is licensed under a Creative Commons Attribution 4.0 International License.
![Scheme 1: The Structure of N,N/-bis (2-hydroxy-3-methoxybenzylidene)-2,2/-(aminophenylthio) ethane [L1] and N,N/-bis (2-hydroxybenzylidene)-2,2/-(aminophenylthio) ethane [L2]](http://www.computerscijournal.org/wp-content/uploads/2008/08/Vol01_No1_Eva_B.A_Sch1-150x150.jpg)




