Asieh Yahyazadeh¹*, Jafar Abbasi², Farshid Salimi and Mohamad Saeed Daneshmandi³
¹Department of Chemistry, University of Guilan, P.O. Box 1914, Rasht (Iran).
²Department of Chemistry, Research Centre Medicinal Plants, Islamic Azad University Ardabil (Iran).
³Department of Electrical and Computer Engineering, University of Tehran, Tehran (Iran).
Article Publishing History
Article Received on :
Article Accepted on :
Article Published :
Article Metrics
ABSTRACT:
Reaction of some diamines and ketones in the presence of catalytic amount of dodecylbenzene sulfonic acid leads to the formation of 2,3–dihydro -1H-1,5-benzodiazepines in good yields. It seems that dodecylbenzene sulfonic acid has two roles: first it acts as a surfactant to dissolve starting materials in water, and second it acts as an acidic catalyst to activate carbonyl groups.
KEYWORDS:
Benzodiazepine; Dodecylbenzene Sulfonic Acid; Imine–Enamine Cyclization
Copy the following to cite this article:
Yahyazadeh A, Abbasi J, Salimi F, Daneshmandi M. S. A New Green Approach to 2,3–Dihydro-1H-1,5 –Benzodiazepines. Orient. J. Comp. Sci. and Technol;2(2)
|
Copy the following to cite this URL:
Yahyazadeh A, Abbasi J, Salimi F, Daneshmandi M. S. A New Green Approach to 2,3–Dihydro-1H-1,5 –Benzodiazepines. Orient. J. Comp. Sci. and Technol;2(2). Available from: http://www.computerscijournal.org/?p=2132
|
Introduction
Benzodiazepines are very important compounds because of their pharmacological properties.1 Despite their importance from a pharmacological point of view, comparatively few methods for the preparation of 1,5- benzodiazepines have been reported. These include condensation reaction of o-phenylenediamines with α,β-unsaturated carbonyl compounds or ketones in the presence of BF3–OEt2, NaBH4, polyphosphoric acid, SiO2, MgO and POCl3.2-6 Unfortunately, many of these processes suffer from one or other limitations such as drastic reaction conditions, low yields and co-occurrence of several side reactions.7-9
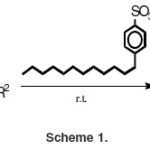
In this work, we report a facile method for the synthesis of 2,3 – dihydro–1H–1,5– benzodiazepines by the condensation of o-phenylenediamine with ketones in the presence of a catalytic amount of dodecylbenzene sulfonic acid in water.
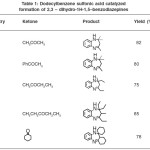
The reactions were carried out in water at room temperature for 12h by taking 1:202 mol ratio mixture of o-phenylenediamine and the ketone in the presence of 1 mol % dodecylbenzene sulfonic acid to give the desired products (Scheme 1).
It is noteworthy that starting from unsymmetrical ketone such as 2-butanone (entry 3), the ring closure occurs selectively only from one side of carbon skeleton yielding a single product. All products were characterized by comparison of their melting point and 1H- NMR spectra with those of authentic samples.
For example, in 1H–NMR spectrum of 2, 2, 4–trimethyl-2,3–dihydro-1H- benzodiazepine (entry 1) in CDCl3 , the CH3 groups showed two singlet at δ=1.34 (6H) and 2.34 (3H) ppm. The CH2 and NH groups appeared at δ= 2.26 (2H) and 3.46 (1H) ppm, respectively. The aromatic protons appeared at δ=6.61-7.28 (4H) ppm, as expected.
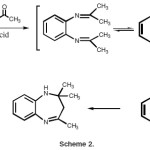
The mechanism of the reaction 2-6 probably involves an intramolecular imine–enamine cyclization as shown in Scheme 2.
In conclusion, a mild and green method has been developed for the synthesis of 2,3–dihydro -1H -1,5–benzodiazepines.
References
- (a) Schutz, H. In Benzodiazepines; Springer: Heidelberg, 2: 240 (1982). (b) Smalley, R. K. In Comprehensive Organic Chemistry, Barton, D., Ollis, W. D., Eds.; Pergamon: Oxford, 4: 600 (1979). (c) Landquist, J. K. In Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Ress, C .W., Eds.; Pergamon: Oxford, l: 166 (1984).
- Stahlofen, P.; Ried, W. Chem. Ber. 90: 815(1957).
- Ried, W.; Torinus, E. Chem. Ber. 90: 2902(1959).
- Herbert, J. A. L.; Suschitzky, H. J. Chem.Soc., Perkin Trans. 2657 (1947).
- Morales, H. R.; Bulbarela, A.; Contreras, R.Heterocycles 24: 135 (1986).
- Jung, D. I, Choi, T. W.; Kim, Y. Y, Kim, I.S.;Park, Y. M.; Lee, Y. G.; Jung, D.H. Synth.Commun. 29 (1941).
- Harmer, M.A., Q., Appel. Catal.General.,221: 45 (2001).
- De, S.K.; Gibbs, R.A.Tetrahedron lett. 46:1811 (2005).
- Nurufe, K.; Ramazan, O. Inorg. Chem. Commun. 11(3): 338 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.