Introduction
A brain–computer interface (BCI), brain–machine interface (BMI), mind-machine interface (MMI), direct neural interface (DNI) is a direct communication between the brain and an external device without any peripheral muscular activity. BCIs are often directed at assisting, augmenting, or repairing human cognitive or sensory-motor functions.
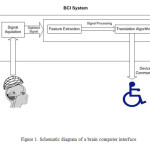
In BCI a subject sends commands to an electronic device through brain activity without any peripheral muscular activity(1-3). Such systems can help patients with motor disabilities (4, 5). To control a BCI, the user should produce various brain activity patterns which are captured in form of Electroencephalogram (EEG) and converted to commands by identifying the patterns by the system. In most BCIs, identification of pattern is based on a classification algorithm, i.e., an algorithm that automatically estimates the class of data represented by a feature vector of the EEG(6-10). Schematic diagram of a typical BCI is presented in figure 1. EEG based BCIs have shown various potentials in different fields including rehabilitation medicine, robotic, space and high technology fields. The main field of EEG-based BCI is developing classification algorithms. Several paradigms have been developed and evaluated for constructing EEG-based BCI systems during the last 20 years(11-18). The paradigms vary a wide range including standard and typical ones such as stimuli-response EEG patterns, biofeedback (BF), to complicated paradigms using sophisticated machine learning algorithms to classify the EEG. Each approach has its advantages and disadvantages. BCI system building paradigms use specific changes in EEG which are induced by controlled external stimuli. These modifications in EEG are called evoked potentials since they are induced by an external stimulus. For example, many BCI systems were built that utilized Steady-State Visually Evoked Potentials (SSVEP) which is elicited by exposing visual stimulus of a box/checkerboard flickering steadily on an LCD screen while the subject gazes on the monitor. A corresponding power increase is identified in the subject’s EEG at the same frequency as well as in the harmonic frequencies of flickering. SSVEP can control a computerized device by flickering many different stimuli at various rates while at the same time allowing a user to shift his/her gaze between different stimuli (19-21). BCI systems operating proved to be effective, with research suggesting that an SSVEP speller system can be constructed which achieves information transfer rates as high as 62.5 bits per minute (bpm) with minimum user training(22-24).
Another evoked potential (EP) measure commonly used for constructing BCI systems is the P300. It is an EP that occurs after presentation of rare-but-expected stimulus. The P300 is called so as it appears in EEG signals as a positive deflection with approximate delay of 300ms following the stimulus onset. An example of BCI system utilizing the P300 is the P300 speller, where a grid of numbers/letters is shown to the user on an LCD screen. Rows and columns of this grid are flashed pseudo-randomly. A P300 is evoked when the user attends to a single character in the grid, as the character flashes infrequently and at intervals which the subject does not know(25). The BCI then determines which character the user attended by tracking each row and column when flashed. Studies reveal that the P300 speller can be successfully used, with information transfer rates as high as 13.3 bpm achieved in subjects with amyotrophic lateral sclerosis and 11.3 bpm in healthy subjects(26). The EP based paradigms have shown great potentials. However, they have some fundamental imitations. As the BCI user has to receive some stimuli, they might be distracted from the tasks they want the computer to perform or the message to be communicated.
Historical Review of BCI
The origin of brain–computer interfaces (BCIs) dated to the discovery of the electrical activity of the human brain and the development of electroencephalography (EEG). In 1924 Berger was the first to record human brain activity by means of EEG. In addition, Berger identified oscillatory activity in the brain by analyzing EEG traces. One wave he identified was alpha wave (8–13 Hz), also known as Berger’s wave.
Berger’s first recording device was immature. He inserted silver wires under the scalps of his patients. These were later replaced by silver foils attached to the patients’ head by rubber bandages. Berger associated the fluctuations in EEG wave with brain diseases. EEGs permitted completely new possibilities for the research of human brain activities. Various studies on the operant conditioning have shown the first time that monkeys could learn to control the deflection of a BF meter arm with neural activity (27). During the 1970, similar studies have demonstrated that monkeys could quickly learn to voluntarily control the firing rates of individual and multiple neurons in the primary motor cortex if they were rewarded for generating appropriate patterns of neural activity (28).
Studies on developing algorithms to reconstruct movements from motor cortexneurons have been started during early 1970s. In the 1980s, a research team at Johns Hopkins University proposed a mathematical relationship, as a cosine function, between the electrical responses of single motor cortex neurons in rhesus macaque monkeys and the direction in which they moved their arms. In addition, this team found that dispersed groups of neurons, in different areas of the monkey’s brains, collectively controlled motor commands, but was able to record the firings of neurons in only one area at a time, because of the technical limitations imposed by his equipment (29).
The mid-1990s has witnessed a dramatic development in BCI technologies (30). Kennedy et al built the first intra-cortical brain–computer BCI by implanting neurotrophic-cone electrodes into monkeys (31).
Yang et al decoded neuronal firings to reproduce images seen by cats (32). They used an array of electrodes embedded in the thalamus of sharp-eyed cats (32). The thalamus main function is relaying and integrating all of the brain’s sensory input.
After conducting initial studies in rats during the 1990s, Nicolelis et al developed BCIs that decoded brain activity in owl monkeys and used the devices to reproduce monkey movements in robotic arms. Monkeys have advanced reaching and grasping abilities and good hand manipulation skills, making them ideal test subjects for such work.
This research team designed a BCI that reproduced owl monkey movements while the monkey operated a joystick or reached for food (33). The BCI operated in real time and could also control a separate robot remotely over Internet protocol. However, the monkeys could not see the arm moving and did not receive any feedback which is called open-loop BCI.
The studies on developing BCI systems have focused on different research avenues. Predicting kinematic and kinetic parameters of limb movements and predicting EMG or electrical activity of the primate muscles are the main fields. In line with these studies, Nicolelis et al demonstrated that the activity of large neural ensembles can predict arm position (34). Lebedev et al argued that brain networks reorganize to create a new representation of the robotic appendage in addition to the representation of the animal’s own limbs [14].
The use of BMIs has also led to a deeper understanding of neural networks and the central nervous system. Studies have shown that despite the belief indicating the highest efficacy of neurons under a group working, single neurons can be conditioned through BMIs to fire at a pattern that allows primates to control motor outputs. In addition, applications of BMIs has led to development of the single neuron insufficiency principle which states that even with a well tuned firing rate single neurons can only carry a narrow amount of information and therefore the highest level of accuracy is achieved by recording firings of the collective ensemble. Other principles discovered with the use of BMIs include the neuronal multitasking principle, the neuronal mass principle, the neural degeneracy principle, and the plasticity principle (34-36).
Technical Principles of BCI
Since the first introduction of EEG in 1929, the idea of using EEG for communication and control where it allows the brain to act on the environment without the normal intermediaries of peripheral nerves and muscles. In the 1970’s, several scientists developed simple communication systems that were driven by electrical activity recorded from the head. Early in that decade, the Advanced Research Projects Agency (ARPA) became interested in technologies that provided a more immersed and intimate interaction between humans and computers and included so-called “bionic” applications. A program proposed by Lawrence focused initially on autoregulation and cognitive BF. It sought to develop BF techniques aiming to improvement of human performance, especially persons with high mental loads including the performance of military personnel engaged in tasks that had high mental loads. The research produced some valuable insights on BF, but made minimal progress toward its stated goals. A new direction, under the more general label of “biocybernetics,” was then defined and became the main source of support for bionics research in the ensuing years. One of the main applications of the bio cybernetics program is using biological signals in the control of vehicles, weaponry, or other systems. The biological signals are analyzed and processed real-time and then are used as a control command for operating remote devices. The most successful project in this area was the system proposed by Vidal et al (37). Using computer-generated visual stimulation and sophisticated signal processing, visual evoked potentials (VEPs) could provide a communication channel through which a human could control the movement of a cursor through a two-dimensional maze (38). The studies on the VEPs as output command for communication have demonstrated that the control systems working with EEG signals are different from the control systems use electromyography (EMG) activity from scalp or facial muscles.
Because scalp-recorded EMG activities are typically much more prominent than EEG activity at the same locations, EMG-based communication can impose significant noises where can wrongly be interpreted as EEG-based communication. To the extent that EMG-based communication is mistaken for EEG-based communication, it can hamper the latter’s development. Detailed spectral and topographical analyses are necessary to distinguish the EMG- from EEG-based signals. In this regard, several studies have been performed to reveal fundamental distinction between EEG-based communication that depends on muscle control (e.g., VEPs that depend on where the eyes are directed), and EEG-based control independent of muscle control.
These distinctions have resulted in new definition of BCI as “a communication system that is independent of the brain’s normal output pathways of peripheral nerves and muscles.” This definition reflects the principal reason for recent interest in BCI development—the possibilities it offers for providing new augmentative communication technology for paralyzed patients or patients with severe movement deficits.
Several different BCIs have been developed that do not depend on nerves and muscles (39)–(40). These systems use either EEG activity recorded from the scalp or the activity of individual cortical neurons recorded from implanted electrodes. While these are exciting developments, with considerable theoretical significance and practical promise, they are relatively low band-width devices, offering maximum information transfer rates of 5–25 bits/min. Furthermore, improvement is likely to be gradual, and to require continued careful and laborious investigation. BCI development requires recognition that a “wire-tapping” analogy probably does not apply—that the goal is not simply to listen in on brain activity , through EEG, intra-cortical recording, or some other method) and thereby determine a person’s intentions.
It is well established that a BCI as a new output channel for the brain can engage the brain’s adaptive capacities that adjust output to optimize performance. Therefore, BCI operation depends on the interaction of two adaptive controllers, the user’s brain, which produces the activity measured by the BCI system, translates that activity into specific commands. Successful BCI operation should consist of proper muscle control as well as proper control of EEG or single-unit activities. Like any communication and control system, a BCI has an input, an output, and a translation algorithm that converts the input into the output. BCI input consists of a particular feature(s) of brain activity and the methodology used to measure that feature(s). BCIs may use frequency-domain features such as EEG or rhythms occurring in specific areas of cortex (41)–(25)(25)–(42), or time-domain features such as slow cortical potentials, P300 potentials, or the action potentials of single cortical neurons (26, 43, 44). The methodology includes the scalp electrode type and locations, the referencing method, the spatial and temporal filters, and other signal processing methods used to detect and measure the features. Nevertheless, the distinction is important because attention to features as reflections of nervous system anatomy and physiology, rather than as merely products of particular analysis methods, helps improvements in BCI technology, and also encourages continued attention to the problem of artifacts such as EMG activity which can affect autoregressive parameters.
Each BCI uses a particular algorithm to translate its input, target EEG features, into output control signals. This algorithm might include linear or nonlinear equations, a neural network, or other methods, and might incorporate continual adaptation of important parameters to key aspects of the input provided by the user(45). BCI outputs can be cursor movement, letter or icon selection, or another form of device control, and provides the feedback that the user and the BCI can use to adapt to optimize communication.
In addition to input, translation algorithm, and output, there are several main characteristics for each BCI has other distinctive characteristics influencing its performance. The main characteristics are On/Off mechanism, response time, speed and accuracy and its integration into information transfer rate, type and extent of required user training, appropriate user population, appropriate applications, and constraints imposed on concurrent conventional sensory input and motor output.
Comparisons of the BCI performance are often difficult because of significant differences in their inputs, translation algorithms, outputs, and other characteristics. Although different systems are required for different applications, developing a standard performance measure as a general purpose benchmark for following BCI development is necessary. A standard measure of communication systems is bit rate, the amount of information communicated per unit time. Bit rate depends on both speed and accuracy (46, 47).For example, the information transfer rate of a BCI that can select between two possible choices with 90% accuracy is twice that of a BCI that can select between them with 80% accuracy, and equal to that of a BCI that can select between four possible choices with 65% accuracy. The enormous importance of accuracy, illustrated by the doubling in information transfer rate with improvement from 80% to 90% accuracy in a two-choice system, has not usually received appropriate recognition in BCI-related publications. While the effectiveness of each BCI system will depend in considerable part on the application to which it is applied, bit rate furnishes an objective measure for comparing different systems and for measuring improvements within systems.
Neurophysiological Signals in BCI
Various signals have been used as measures in BCI systems. Interfaces based on brain signals require on-line detection of mental states from spontaneous activity: different cortical areas are activated while thinking different things such as a mathematical computation, an imagined arm movement, a music composition, etc. The information of these “mental states” can be recorded with different methods. Neuropsychological signals can be generated by one or more of the following three approached: implanted methods, evoked potentials, known as event related potentials (ERP), and operant conditioning. Both EP and operant conditioning methods are normally externally-based BCIs as the electrodes are located on the scalp. Table 1 describes the different signals used in BCIs. However, some of the described signals fit into multiple categories. As an example, single neural recordings may use operant conditioning to train neurons for control or may accept the natural occurring signals for control(8, 42, 48-51).
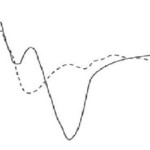
Implanted methods use signals from single or small groups of neurons to control a BCI. In most cases, the most suitable option for placing the electrodes is the motor cortex region, because of its direct relevance to motor tasks, its relative accessibility compared to motor areas deeper in the brain, and the relative ease of recording from its large pyramidal cells. These methods can result in relatively high signal-to-noise ratio, however they are invasive. They require no remaining motor control and may provide either discrete or continuous control. While most systems are still in the experimental stage, Kennedy’s group has forged ahead to provide control for locked-in patient JR (31, 52, 53). Kennedy’s approach involves encouraging the growth of neural tissue into the hollow tip of a two-wire electrode known as a neurotrophic electrode. The tip contains growth factors that spur brain tissue to grow through it. Through an amplifier and antennas positioned between the skull and the scalp, the neural signals are transmitted to a computer, which in turn uses the signals to drive a mouse cursor. This technique has provided stable long term recording and patient JR has learned to produce synthetic speech with the BCI over a period of more than 426 days. The efficacy of this technique on multiple individuals is not clear, but it has EPs are brain potentials that are evoked by the occurrence of a sensory stimulus. They are usually obtained by averaging a number of brief EEG segments time-registered to a stimulus in a simple task. In a BCI, EPs may provide control when the BCI application produces the appropriate stimuli. This paradigm has the benefit of requiring little to no training to us e the BCI at the cost of having to make users wait for the relevant stimulus presentation. EPs offer discrete control for almost all users, as EPs are an inherent response. Exogenous components, or those components influenced primarily by physical stimulus properties, generally take place within the first 200 milliseconds after stimulus onset. These components include a Negative waveform around 100 ms (N1) and a positive waveform around 200 ms after stimulus onset (P2). VEPs fall into this category. Sutter uses short visual stimuli in order to determine what command an individual is looking at and therefore wants to pick He also shows that implanting electrodes improves performance of an externally-based BCI. In a different approach, McMillan and colleagues have trained volunteers to control the amplitude of their steady-state VEPs to florescent tubes flashing at 13.25 Hz(54-56). Using VEPs has the benefit of a quicker response than longer latency components. The VEP requires subject to have good visual control in order to look at the appropriate stimulus and allows for discrete control. As the VEP is an exogenous component, it should be relatively stable over time. Endogenous components, or those components influenced by cognitive factors, take place following the exogenous components. Different research teams independently discovered a wave peaking at around 300 ms after task-relevant stimuli (57, 58). This component is known as the P3 (Fig 2). While the P3 is evoked by many types of paradigms, the most common factors influencing it are stimulus frequency (less frequent stimuli produce a larger response) and task relevance. The P3 is fairly stable in locked-in patients, re-appearing even after severe brain stem injuries. Farwell and Donchin first showed that this signal may be successfully used in a BCI (59). Using a broad cognitive signal like the P3 has the benefit of enabling control through a variety of modalities, as the P3 enables discrete control in response to both auditory and visual stimuli. P3 is a cognitive component and reportedly changed in response to subject’s fatigue.
Operant conditioning is a method for modifying the behavior (an operant), which utilizes contingencies between a discriminative stimulus, an operant response, and a reinforcer to change the probability of a response occurring again in a given situation. In the BCI framework, it is used to train the patients to control their EEG. As it is presented in Table 3.1, several methods use operant conditioning on spontaneous EEG signals for BCI control. The main feature of this kind of signals is that it enables continuous rather than discrete control. This feature may also serve as a drawback: continuous control is fatiguing for subjects and fatigue may cause changes in performance since control is learned.
Wolpaw et al train individuals to control their Mu wave amplitude for cursor control (60). Mu wave control does not require subjects to have any remaining motor control. For the cursor control task, normal subjects are trained on the order of 10-15 sessions to learn to move the cursor up/down. In the several papers examined, it appears that not all subjects obtain control, although most seem to during this time frame. In related work, the Graz brain-computer interface trains people to control the amplitude of their ERS/ERD patterns. Subjects are trained over a few sessions in order to learn a cursor control task. As in the Mu wave control, not all subjects learn to control the cursor accurately. One of the interesting aspects of this system is that it gives BF to the user in the form of a moving cursor after training. Several studies have shown that EEG signal shows different characteristics during different mental calculations. These results indicate that different regions of the brain are active during different types of mental calculation, and if these different tasks may be accurately recognized, they could be used in a BCI. Lin et al. (61) describe a study where five tasks were compared: multiplication problem solving, geometric figure rotation, mental letter composing, visual counting, and a baseline task where the subject was instructed to think about nothing in particular. The results of this experiment suggest that the easiest tasks to identify are multiplication problem solving and geometric figure rotation, but even these tasks are not easily identified. Other papers have concentrated on mental tasks, but none have found easily recognizable differences between different tasks(62). BCI uses different strategies to control the output the main of them are motor imagery, BF or NF and visual evoked potential.
Neurogaming
Neurogaming is a new field of gaming that uses non-invasive BCI to improve game-play so that users can interact with a console without the use of a traditional controller (63). Some neurogaming software use a player’s brain waves, heart rate, expressions, pupil dilation, and even emotions to complete tasks or affect the mood of the game[Schwarz, 2014 #375]. For example, game developers at Emotiv have created non-invasive BCI that will determine the mood of a player and adjust music or scenery accordingly. This new form of interaction between player and software will enable a player to have a more realistic gaming experience. Because there will be less disconnect between a player and console, Neurogaming will allow individuals to utilize their “psychological state and have their reactions transfer to games in real-time (64). However, neurogaming is a relatively new technology which need more detailed and controlled studies to develop reliable technique.
Motor Imagery
Motor imagery involves the imagination of the movement of various body parts resulting in sensorimotor cortex activation, which modulates sensorimotor oscillations in the EEG. This can be detected by the BCI to infer a user’s intent. Motor imagery requires a number of sessions of training before acceptable control of the BCI is acquired. These training sessions may take a number of hours over several days before users can consistently employ the technique with acceptable levels of precision. Regardless of the duration of the training session, users are unable to master the control scheme. This results in very slow pace of the game-play.
Biofeedback/ Neurofeedback
BF is used to monitor a subject’s mental relaxation. In some cases, BF does not monitor EEG, but instead bodily parameters such as (EMG), galvanic skin resistance (GSR), and heart rate variability (HRV). Many BF systems are used to treat certain disorders such as attention deficit hyperactivity disorder (ADHD), sleep problems in children, teeth grinding, and chronic pain. EEG BF, also known as neurofeedback (NF) systems typically monitor four different bands (theta: 4–7 Hz, alpha:8–12 Hz, SMR: 12–15 Hz, beta: 15–18 Hz) and direct the subject to control these waves towards desirable states. Passive BCI involves using BCI to enrich human–machine interaction with implicit information on the actual user’s state. Using simulations to detect when users intend to push brakes during an emergency car stopping procedure is an example of passive BCI. Game developers using passive BCIs need to acknowledge that through repetition of game levels the user’s cognitive state will change or adapt. Within the first play of a level, the user will react to things differently during the second play: for example, the user will be less surprised at an event in the game if he/she is expecting it (65).
Visual Evoked Potential
A VEP is an electrical potential recorded after a subject is exposed by a visual stimulus. There are several types of VEPs.
Steady-state visually evoked potentials (SSVEPs) use potentials generated by exciting the retina, using visual stimuli modulated at certain frequencies. SSVEPs stimuli are often formed from alternating checkerboard patterns or flashing images. The frequency of the phase reversal of the stimulus is clearly distinguished in the EEG spectrum which in turn the SSVEP stimuli is easily detected. Several studies have shown the efficacy of SSVEP in several BCI systems. Elicited SSVEP has high amplitude so that the transient VEP and blink movement and ECG artifacts do not affect the frequencies monitored. In addition, the SSVEP signal is highly robust where the topographic organization of the primary visual cortex is such that a broader area obtains afferents from the central region of the visual field. However, SSVEP has several problems. As SSVEPs use flashing stimuli to infer a user’s intent, the user must gaze at one of the flashing or iterating symbols to interact with the system. It is, therefore, likely that the symbols could become irritating and uncomfortable to use during longer play sessions, which can often last more than an hour which may not be an ideal gameplay.
Another type of VEP used in BCIs the P300 potential. The P300 event-related potential is a positive peak in the EEG that occurs at roughly 300 ms after the appearance of a target or oddball stimulus. The P300 is reportedly related to a higher level attention process or an orienting response. Using P300 as a control scheme in BCIs has the advantage of needing short training sessions. The first application of P300 measure as control in BCI is the P300 matrix. In this system, a subject chooses a letter from a grid of 6 by 6 letters and numbers. The rows and columns of the grid flashed sequentially and every time the selected “choice letter” illuminates the user’s P300 is potentially elicited. However, the communication process, at approximately 17 characters per minute is quite slow. The P300 is a discrete rather than a continuous control mechanism. The advantage of P300 in play gaming is that the player does not have to teach himself/herself how to use a completely new control system but only has to undertake short training instances, to learn the gameplay mechanics and basic use of the BCI paradigm.
Invasive BCIs
Two different research approaches have resulted in two different types of BCIs: invasive BCIs, characterized by implanted electrodes in brain tissue and noninvasive BCIs which work on electrophysiological recordings in humans such as EEG and magnetoencephalography (MEG) and metabolic changes such as functional magnetic resonance imaging (fMRI) and near infrared spectroscopy (NIRS). Both invasive and non-invasive BCIs have originated from animal experiments. Invasive BCIs consist of implanted multi-electrode arrays in the motor cortex of paralyzed patients, premotor cortex of monkeys, or parietal motor command areas (34, 66, 67). In invasive BCIs intended skilled movements are reconstructed from neuronal firing patterns on-line. These reconstructions use different approaches Different On the basis of “sparse coding” approaches to motor learning (Riehle and Vaaida, 2005) and directional coding vectors of motor neurons (Georgopulos et al., 1986), automatized complex movements can be reconstructed on-line from relatively few motor neurons using simple algorithms: Nicolelis’ group (Carmena et al., 2003) demonstrated in monkeys after extensive training of a reaching and grasping movement that firing patterns of 32 neurons are sufficient to execute that movement directly with an artificial limb. Chapin et al. (1999) trained rats to move a lever with an artificial arm in a Skinner box for reward with extracellular firing of cortical cells without any actual movement.
The second category of BCI research is originated in the concepts of BF and instrumental-operant learning of autonomic functions. During the late 1960s and early 1970s Miller et al opposed the traditional wisdom of the autonomous nervous system (ANS) as autonomous, independent of voluntary control of the somatic central nervous system (CNS). Voluntary control is acquired through operant (instrumental) conditioning, whereas modification of involuntary ANS functions is learned through classical (Pavlovian) conditioning, a distinction first emphasized by Skinner, 1953; Holland & Skinner, 1961.
Miller (1969) challenged that view and presented experimental evidence in curarized and artificially ventilated rats: even after long-term curarization of several weeks the animals learned to increase and decrease heart rate, renal blood flow, dilation and constriction of peripheral arteries in an operant conditioning paradigm rewarding the animals for increases and decreases of the particular physiological function. These studies stirred an enormous interest in the scientific and clinical community, particularly in psychosomatic medicine and behavior modification. The results suggested that instrumental (“voluntary”) control of autonomic functions is possible without any mediation of the somatic-muscular system paralyzed by curarization: heart rate increase is usually learned and controlled by an increase in muscle tension; curarization prevents this mediation of the motor system. Various studies have shown that operant training of any internal body function is possible. As a result, various research interests have been stimulated to seek treat different diseases such as high blood pressure, cardiac arrhythmias, vascular pathologies, renal failure, gastrointestinal disorders, and many others.
Vision
Invasive BCI techniques aim at repairing damaged sight and providing new functionality for people with paralysis. Invasive BCIs are implanted directly into the grey matter of the brain during neurosurgery. The invasive devices produce the highest quality signals among BCI devices since they are implanted into the grey matter. However, they are associated with the risk of scar-tissue build-up, weakening or even eliminating the signal as the body reacts to a foreign object in the brain (37).
In vision science, direct brain implants have been used to treat non-congenital blindness (68). Dobelle et al proposed the first working brain interface to restore sight. Dobelle’s first prototype was implanted into “Jerry”, a man blinded in adulthood, in 1978. A single-array BCI containing 68 electrodes was implanted onto Jerry’s visual cortex and succeeded in producing the sensation of seeing light, phosphenes . The system included cameras mounted on glasses to send signals to the implant. Initially, the implant allowed Jerry to see shades of grey in a limited field of vision at a low frame-rate. This also required him to be hooked up to a mainframe computer, but shrinking electronics and faster computers made his artificial eye more portable and now enable him to perform simple tasks unassisted (69).
Movement
BCIs focusing on motor neuroprosthetics aim to either restore movement in individuals with paralysis or provide devices to assist them, such as interfaces with computers or robot arms.
Researchers at Emory University in Atlanta, were first to install a brain implant in a human that produced signals of high enough quality to simulate movement. Their patient, Johnny Ray (1944–2002), suffered from ‘locked-in syndrome’ after suffering a brain-stem stroke in 1997. Ray’s implant was installed in 1998 and he lived long enough to start working with the implant, eventually learning to control a computer cursor; he died in 2002 of a brain aneurysm(70).
Matt Nagle who was paralyzed from the neck down, became the first person to control an artificial hand using a BCI in 2005. A 96-electrode chip was implanted in his right precentral gyrus, the area of the motor cortex for arm movement. The implant allowed Nagle to control a robotic arm by thinking about moving his hand as well as a computer cursor, lights and TV(71). This was the first BCI with electrodes located on the surface of the skull, instead of directly in the brain.
More recently, several studies have demonstrated further success in direct control of robotic prosthetic limbs with high degree of freedom using direct connections to arrays of neurons in the motor cortex of patients with tetraplegia (72, 73).
Partially Invasive BCIs
Partially invasive BCI devices are implanted inside the skull but rest outside the brain rather than within the grey matter. They produce better resolution signals than non-invasive BCIs where the bone tissue of the cranium deflects and deforms signals with lower risk of scar-tissue forming in the brain than fully invasive BCIs.
Electrocorticography (ECoG) measures the electrical activity of the brain from beneath the skull so that the electrodes are placed above the cortex, beneath the durra mater (74). ECoG technologies were first trialled in humans in 2004. In a later trial, the researchers enabled a teenage boy to play Space Invaders using his ECoG implant (75). This research indicates that control is rapid, requires minimal training, and may be an ideal tradeoff with regards to signal fidelity and level of invasiveness.
ECoG is a very promising intermediate BCI modality because it has higher spatial resolution, better signal-to-noise ratio, wider frequency range, and less training requirements than scalp-recorded EEG, while lower technical difficulty, lower clinical risk, and superior long-term stability than intra-cortical single-neuron recording (74). The control outputs extracted from ECOG signal enjoy high level of control with minimal training requirements. Recent studies have shown great potentials of ECOG measures as control signal for BCIs especially for patients with severe motor disabilities shows potential for real world application for people with motor(76, 77).
Non-Invasive BCIs
In non-invasive BCIs non-invasive neuro-imaging technologies are used as interfaces. Signals recorded in these techniques are used to power muscle implants and restore partial movement in an experimental volunteer. Although they are easy to wear, non-invasive implants produce poor signal resolution because the skull dampens signals, dispersing and blurring the electromagnetic waves created by the neurons. Although the waves can still be detected, determining the brain’s region produces the waves is highly difficult (78-82).
EEG Based Classification in BCI
It is well known that the variation of the surface potential distribution on the scalp reflects functional activities emerging from the underlying brain (83). This surface potential variation can be recorded by affixing an array of electrodes to the scalp, and measuring the voltage between pairs of these electrodes, which are then filtered, amplified, and recorded. The resulting data is called the EEG.
EEG is the most studied potential non-invasive interface, mainly because of its fine temporal resolution, ease of use, portability and low set-up cost. However, An EEG recording system is highly susceptible to noise. Another substantial barrier to using EEG as a BCI is the extensive training a user is required to work with the system. For example, in experiments which were aimed to train severely paralyzed people to self-regulate the slow cortical potentials in their EEG to such an extent that these signals could be used as a binary (84). The experiment showed ten patients trained to move a computer cursor by controlling their brainwaves. The process was slow, requiring more than an hour for patients to write 100 characters with the cursor, while training often took many months.
Oscillatory activity is another parameter with high control value in a BCI system. Users can choose the brain signals to operate a BCI, including mu and Beta wave is one of these brain waves.
Patterns of P300 waves are generated involuntarily (stimulus-feedback) when people see something they recognize and may allow BCIs to decode categories of thoughts without training patients first. By contrast, the BF methods described above require learning to control brainwaves so the resulting brain activity can be detected.
Lawrence Farwell and Emanuel Donchin developed an EEG-based BCI in the 1980s (59). Their “mental prosthesis” used the P300 brainwave response to allow subjects, including one paralyzed Locked-In syndrome patient, to communicate words, letters and simple commands to a computer and thereby to speak through a speech synthesizer driven by the computer.
Recent advances in EEG signal processing, new insights into association between EEG-based measures and mental states and advances in high resolution EEG measurements have suggested the efficacy of EEG-based BCI comparable with invasive BCI. Using advanced functional neuro-imaging such as BOLD functional MRI and EEG source imaging, co-variation and co-localization of electrophysiological and hemodynamic signals induced by motor imagination are possible (85).
In addition to a brain-computer interface based on brain waves, as recorded from scalp EEG electrodes, virtual EEG signal-based BCI using the EEG inverse problem solution has been investigated in different studies(86).
Neurogaming
Neurogaming is a new field of gaming that uses non-invasive BCI to improve game-play so that users can interact with a console without the use of a traditional controller(63). Some neurogaming software use a player’s brain waves, heart rate, expressions, pupil dilation, and even emotions to complete tasks or affect the mood of the game(87). For example, game developers at Emotiv have created non-invasive BCI that will determine the mood of a player and adjust music or scenery accordingly. This new form of interaction between player and software will enable a player to have a more realistic gaming experience. Because there will be less disconnect between a player and console, Neurogaming will allow individuals to utilize their “psychological state and have their reactions transfer to games in real-time (64). However, neurogaming is a relatively new technology which need more detailed and controlled studies to develop reliable technique.
Future Directions
One of the main issues in BCI is the dependence of its operation on the user behaviors in encoding his/her desires in the EEG features that the system measures and translates into output control signals. Therefore, the progress depends on development of improved training methods. Future studies should evaluate the effects of the instructions given to users, and analyze the relationships between user reports of strategies employed and actual BCI performance. For example, some BCI protocols ask the user to employ very specific motor imagery such as imagery of right or left hand movement or other mental tasks to produce the EEG features the system uses as control signals (41, 88), (89). Others may leave the choice of imagery or even using any imagery up to the user (41), (90). Analysis of the similarities and differences between acquisition of BCI control and acquisition of conventional motor or non-motor skills is necessary to improving the training methods. In addition, the possible impacts of subject mental states including motivation, fatigue, frustration, and other aspects require exploration. Users’ reports might help in assessing these factors. However, the value of user’s reports is controversial. Users’ reports of their strategies may not accurately reflect the processes of achieving and maintaining EEG control (91).
Light reactive imaging BCI technology is one of the most recent advances in BCI technology. This technique involves implanting a laser inside the skull. The laser is trained on a single neuron and the neuron’s reflectance is measured by a separate sensor. Upon the firing of the neuron, pattern and wavelengths of the reflected laser light slightly change. This would allow researchers to monitor single neurons but requires less contact with tissue and reduce the risk of scar-tissue build-up. The most important barrier in developing BCI technology is currently the lack of a sensor modality that provides safe, accurate and robust access to brain signals. However, developing such as sensor is achievable within the next twenty years. Application of such sensor can greatly expand the range of communication functions that can be provided using a BCI.
The continuation and acceleration of BCI development and application does not depend solely on scientific and technical advances. It depends also on attention to important practical issues including the healthcare strategies and appropriate funding. Nowadays, the development of BCI technique is limited by limited funding and small number of people involved. On the other hand, naive and overly enthusiastic media attention is likely to be detrimental in the long run. Major funding increases, particularly for development of specific applications, depend on generating interest from industry and on securing approval for reimbursement from medical insurance companies. Industrial interest depends in large measure on the numbers of potential users. Expansion beyond the relatively small numbers of people, who are locked-in, to include individuals with high-level spinal cord injuries or severe cerebral palsy, could draw much greater commercial interest. Furthermore, widespread application of BCI-based communication systems depends on cost, ease of training and use, as well as on careful attention to user satisfaction.
Conclusion
The studies on BCIs have shown promising potentials. A BCI is a communication and control channel without the brain’s normal output path-ways of peripheral nerves and muscles. Any BCI system has inputs, outputs, and translation algorithms converting the input to the output. BCI operation depends on the interaction of the user’s brain and the system itself. A successful development of BCI system needs close interdisciplinary cooperation between neuroscientists, engineers, psychologists, computer scientists, and rehabilitation specialists. Further development in designing precise sensors capable of recording the brain’s signals in single unit level is crucial step in BCI development.
References
- Wolpaw JR, McFarland DJ, Neat GW, Forneris CA. An EEG-based brain-computer interface for cursor control. Electroencephalography and clinical neurophysiology. 1991;78(3):252-9.
CrossRef
- Pfurtscheller G, Flotzinger D, Pregenzer M, Wolpaw JR, McFarland D. EEG-based brain computer interface (BCI). Search for optimal electrode positions and frequency components. Medical progress through technology. 1995;21(3):111-21.
- Kalcher J, Flotzinger D, Neuper C, Golly S, Pfurtscheller G. Graz brain-computer interface II: towards communication between humans and computers based on online classification of three different EEG patterns. Medical & biological engineering & computing. 1996;34(5):382-8.
CrossRef
- Cincotti F, Aloise F, Bufalari S, Schalk G, Oriolo G, Cherubini A, et al. Non-invasive brain-computer interface system to operate assistive devices. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2007;2007:2532-5.
CrossRef
- Frolov AA, Biriukova EV, Bobrov PD, Mokienko OA, Platonov AK, Prianichnikov VE, et al. [Principles of neurorehabilitation based on brain-computer interface and biologically plausible control of the exoskeleton]. Fiziologiia cheloveka. 2013;39(2):99-113.
CrossRef
- Gerjets P, Walter C, Rosenstiel W, Bogdan M, Zander TO. Cognitive state monitoring and the design of adaptive instruction in digital environments: lessons learned from cognitive workload assessment using a passive brain-computer interface approach. Frontiers in neuroscience. 2014;8:385.
CrossRef
- Goksu F, Ince NF, Tadipatri VA, Tewfik AH. Classification of EEG with structural feature dictionaries in a brain computer interface. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2008;2008:1001-4.
CrossRef
- Kashihara K. A brain-computer interface for potential non-verbal facial communication based on EEG signals related to specific emotions. Frontiers in neuroscience. 2014;8:244.
CrossRef
- Kauhanen L, Jylanki P, Lehtonen J, Rantanen P, Alaranta H, Sams M. EEG-based brain-computer interface for tetraplegics. Computational intelligence and neuroscience. 2007:23864.
CrossRef
- Zhang H, Yang H, Guan C. Bayesian learning for spatial filtering in an EEG-based brain-computer interface. IEEE transactions on neural networks and learning systems. 2013;24(7):1049-60.
CrossRef
- Guger C, Edlinger G, Harkam W, Niedermayer I, Pfurtscheller G. How many people are able to operate an EEG-based brain-computer interface (BCI)? IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2003;11(2):145-7.
CrossRef
- Gysels E, Celka P. Phase synchronization for the recognition of mental tasks in a brain-computer interface. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2004;12(4):406-15.
CrossRef
- Hazrati M, Erfanian A. An online EEG-based brain-computer interface for controlling hand grasp using an adaptive probabilistic neural network. Medical engineering & physics. 2010;32(7):730-9.
CrossRef
- He W, Wei P, Zhou Y, Wang L. Combination of amplitude and phase features under a uniform framework with EMD in EEG-based Brain-Computer Interface. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2012;2012:1687-90.
- Herman P, Prasad G, McGinnity TM. Investigation of the Type-2 Fuzzy Logic Approach to Classification in an EEG-based Brain-Computer Interface. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2005;5:5354-7.
CrossRef
- Ince NF, Tadipatri VA, Goksu F, Tewfik AH. Denoising of multiscale/multiresolution structural feature dictionaries for rapid training of a brain computer interface. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2009;2009:21-4.
CrossRef
- Ince NF, Tewfik AH, Arica S. A space-time-frequency analysis approach for the classification motor imagery EEG recordings in a brain computer interface task. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2006;1:2581-4.
CrossRef
- Kamousi B, Liu Z, He B. Classification of motor imagery tasks for brain-computer interface applications by means of two equivalent dipoles analysis. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2005;13(2):166-71.
CrossRef
- Gollee H, Volosyak I, McLachlan AJ, Hunt KJ, Graser A. An SSVEP-based brain-computer interface for the control of functional electrical stimulation. IEEE transactions on bio-medical engineering. 2010;57(8):1847-55.
CrossRef
- Lee PL, Sie JJ, Liu YJ, Wu CH, Lee MH, Shu CH, et al. An SSVEP-actuated brain computer interface using phase-tagged flickering sequences: a cursor system. Annals of biomedical engineering. 2010;38(7):2383-97.
CrossRef
- Lin YP, Wang Y, Jung TP. A mobile SSVEP-based brain-computer interface for freely moving humans: the robustness of canonical correlation analysis to motion artifacts. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2013;2013:1350-3.
- Han CH, Hwang HJ, Lim JH, Im CH. Development of an “eyes-closed” brain-computer interface system for communication of patients with oculomotor impairment. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2013;2013:2236-9.
- Luo A, Sullivan TJ. A user-friendly SSVEP-based brain-computer interface using a time-domain classifier. Journal of neural engineering. 2010;7(2):26010.
CrossRef
- Wu CH, Chang HC, Lee PL, Li KS, Sie JJ, Sun CW, et al. Frequency recognition in an SSVEP-based brain computer interface using empirical mode decomposition and refined generalized zero-crossing. Journal of neuroscience methods. 2011;196(1):170-81.
CrossRef
- Aloise F, Schettini F, Arico P, Leotta F, Salinari S, Mattia D, et al. P300-based brain-computer interface for environmental control: an asynchronous approach. Journal of neural engineering. 2011;8(2):025025.
CrossRef
- Mak JN, McFarland DJ, Vaughan TM, McCane LM, Tsui PZ, Zeitlin DJ, et al. EEG correlates of P300-based brain-computer interface (BCI) performance in people with amyotrophic lateral sclerosis. Journal of neural engineering. 2012;9(2):026014.
CrossRef
- Fetz EE. Operant conditioning of cortical unit activity. Science. 1969;163(3870):955-8.
CrossRef
- Schmidt E, McIntosh J, Durelli L, Bak M. Fine control of operantly conditioned firing patterns of cortical neurons. Experimental neurology. 1978;61(2):349-69.
CrossRef
- PETRIDES M, SCHWARTZ AB, MASSEY J. Mental Rotation of the Neuronal Populaton Vector. Science. 1989;243:234.
CrossRef
- Lebedev MA, Nicolelis MA. Brain–machine interfaces: past, present and future. TRENDS in Neurosciences. 2006;29(9):536-46.
CrossRef
- Kennedy PR, Adams KD. A decision tree for brain-computer interface devices. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2003;11(2):148-50.
CrossRef
- Stanley GB, Li FF, Dan Y. Reconstruction of natural scenes from ensemble responses in the lateral geniculate nucleus. The Journal of Neuroscience. 1999;19(18):8036-42.
- Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, Chapin JK, et al. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 2000;408(6810):361-5.
CrossRef
- Carmena JM, Lebedev MA, Crist RE, O’Doherty JE, Santucci DM, Dimitrov DF, et al. Learning to control a brain–machine interface for reaching and grasping by primates. PLoS biology. 2003;1(2):e42.
CrossRef
- Furdea A, Ruf CA, Halder S, De Massari D, Bogdan M, Rosenstiel W, et al. A new (semantic) reflexive brain-computer interface: in search for a suitable classifier. Journal of neuroscience methods. 2012;203(1):233-40.
CrossRef
- Kaplan AY, Lim JJ, Jin KS, Park BW, Byeon JG, Tarasova SU. Unconscious operant conditioning in the paradigm of brain-computer interface based on color perception. The International journal of neuroscience. 2005;115(6):781-802.
CrossRef
- Vidal JJ. Real-time detection of brain events in EEG. Proceedings of the IEEE. 1977;65(5):633-41.
CrossRef
- Orhan U, Erdogmus D, Roark B, Purwar S, Hild KE, 2nd, Oken B, et al. Fusion with language models improves spelling accuracy for ERP-based brain computer interface spellers. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2011;2011:5774-7.
CrossRef
- Angelakis E, Hatzis A, Panourias IG, Sakas DE. Brain-computer interface: a reciprocal self-regulated neuromodulation. Acta neurochirurgica Supplement. 2007;97(Pt 2):555-9.
- Bobrov P, Frolov A, Cantor C, Fedulova I, Bakhnyan M, Zhavoronkov A. Brain-computer interface based on generation of visual images. PloS one. 2011;6(6):e20674.
CrossRef
- Ahn M, Hong JH, Jun SC. Feasibility of approaches combining sensor and source features in brain-computer interface. Journal of neuroscience methods. 2012;204(1):168-78.
CrossRef
- Jiang J, Zhou Z, Yin E, Yu Y, Hu D. Hybrid Brain-Computer Interface (BCI) based on the EEG and EOG signals. Bio-medical materials and engineering. 2014;24(6):2919-25.
- Mirghasemi H, Fazel-Rezai R, Shamsollahi MB. Analysis of p300 classifiers in brain computer interface speller. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2006;1:6205-8.
CrossRef
- Wang J, Yang C. [Research of controlling of smart home system based on P300 brain-computer interface]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2014;31(4):762-6.
- McFarland DJ, Sarnacki WA, Vaughan TM, Wolpaw JR. Brain-computer interface (BCI) operation: signal and noise during early training sessions. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2005;116(1):56-62.
CrossRef
- Obermaier B, Neuper C, Guger C, Pfurtscheller G. Information transfer rate in a five-classes brain-computer interface. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2001;9(3):283-8.
CrossRef
- Krausz G, Scherer R, Korisek G, Pfurtscheller G. Critical decision-speed and information transfer in the “Graz Brain-Computer Interface”. Applied psychophysiology and biofeedback. 2003;28(3):233-40.
CrossRef
- Mayaud L, Congedo M, Van Laghenhove A, Orlikowski D, Figere M, Azabou E, et al. A comparison of recording modalities of P300 event-related potentials (ERP) for brain-computer interface (BCI) paradigm. Neurophysiologie clinique = Clinical neurophysiology. 2013;43(4):217-27.
CrossRef
- Wang L, Wang S, Kuang G. [A study of brain-computer interface paradigm based on mental arithmetic]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2013;30(3):469-75.
- Muller-Putz GR, Daly I, Kaiser V. Motor imagery-induced EEG patterns in individuals with spinal cord injury and their impact on brain-computer interface accuracy. Journal of neural engineering. 2014;11(3):035011.
CrossRef
- Ono T, Shindo K, Kawashima K, Ota N, Ito M, Ota T, et al. Brain-computer interface with somatosensory feedback improves functional recovery from severe hemiplegia due to chronic stroke. Frontiers in neuroengineering. 2014;7:19.
CrossRef
- Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain–computer interfaces for communication and control. Clinical neurophysiology. 2002;113(6):767-91.
CrossRef
- Kennedy PR, Bakay RA, Moore MM, Adams K, Goldwaithe J. Direct control of a computer from the human central nervous system. Rehabilitation Engineering, IEEE Transactions on. 2000;8(2):198-202.
CrossRef
- Jones KS, Middendorf MS, Calhoun G, McMillan G, editors. Evaluation of an electroencephalographic-based control device. Proceedings of the Human Factors and Ergonomics Society Annual Meeting; 1998: SAGE Publications.
CrossRef
- Middendorf M, McMillan G, Calhoun G, Jones KS. Brain-computer interfaces based on the steady-state visual-evoked response. IEEE Transactions on Rehabilitation Engineering. 2000;8(2):211-4.
CrossRef
- Zhang Y, Zhou G, Jin J, Wang X, Cichocki A. SSVEP recognition using common feature analysis in brain-computer interface. Journal of neuroscience methods. 2014.
- Chapman RM, Bragdon HR. Evoked responses to numerical and non-numerical visual stimuli while problem solving. 1964.
- Sutton S, Braren M, Zubin J, John E. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150(3700):1187-8.
CrossRef
- Farwell LA, Donchin E. Talking off the top of your head: toward a mental prosthesis utilizing event-related brain potentials. Electroencephalography and clinical neurophysiology. 1988;70(6):510-23.
CrossRef
- Wolpaw JR, McFarland DJ, Vaughan TM. Brain-computer interface research at the Wadsworth Center. IEEE transactions on rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2000;8(2):222-6.
CrossRef
- Lin S-L, Tsai Y-J, Liou C-Y. Conscious mental tasks and their EEG signals. Medical and biological engineering and computing. 1993;31(4):421-6.
CrossRef
- Fernández T, Harmony T, Rodríguez M, Bernal J, Silva J, Reyes A, et al. EEG activation patterns during the performance of tasks involving different components of mental calculation. Electroencephalography and clinical neurophysiology. 1995;94(3):175-82.
CrossRef
- Raphael G, Behneman A, Tan V, Pojman N, Berka C. Interactive Neuro-Educational Technologies (I-NET): Development of a novel platform for neurogaming. Foundations of Augmented Cognition Directing the Future of Adaptive Systems: Springer; 2011. p. 452-61.
CrossRef
- Berka C, Pojman N, Trejo J, Coyne J, Cole A, Fidopiastis C, et al. Merging Cognitive Neuroscience & Virtual Simulation in an Interactive Training Platform.
- Marshall D, Coyle D, Wilson S, Callaghan M. Games, gameplay, and BCI: The state of the art. Computational Intelligence and AI in Games, IEEE Transactions on. 2013;5(2):82-99.
CrossRef
- Donoghue JP. Connecting cortex to machines: recent advances in brain interfaces. nature neuroscience. 2002;5:1085-8.
CrossRef
- Schwartz AB, Taylor DM, Tillery SIH. Extraction algorithms for cortical control of arm prosthetics. Current opinion in neurobiology. 2001;11(6):701-8.
CrossRef
- Naumann J. Search for Paradise: A Patient’s Account of the Artificial Vision Experiment: Xlibris Corporation; 2012.
- Foster S. Book of vision quest: Simon and Schuster; 1989.
- Kennedy PR, Bakay RA. Restoration of neural output from a paralyzed patient by a direct brain connection. Neuroreport. 1998;9(8):1707-11.
CrossRef
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442(7099):164-71.
CrossRef
- Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485(7398):372-5.
CrossRef
- Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, et al. High-performance neuroprosthetic control by an individual with tetraplegia. The Lancet. 2013;381(9866):557-64.
CrossRef
- Schalk G, Miller K, Anderson N, Wilson J, Smyth M, Ojemann J, et al. Two-dimensional movement control using electrocorticographic signals in humans. Journal of neural engineering. 2008;5(1):75.
CrossRef
- Fitzpatrick T. Teenager moves video icons just by imagination. Washington University in St Louis, Available at:[http://news wustl edu/news/Pages/7800 aspx], Accessed at. 2012;10(03).
- Pei X, Barbour DL, Leuthardt EC, Schalk G. Decoding vowels and consonants in spoken and imagined words using electrocorticographic signals in humans. Journal of neural engineering. 2011;8(4):046028.
CrossRef
- Yanagisawa T, Hirata M, Saitoh Y, Kishima H, Matsushita K, Goto T, et al. Electrocorticographic control of a prosthetic arm in paralyzed patients. Annals of neurology. 2012;71(3):353-61.
CrossRef
- Blankertz B, Dornhege G, Krauledat M, Muller KR, Kunzmann V, Losch F, et al. The Berlin Brain-Computer Interface: EEG-based communication without subject training. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2006;14(2):147-52.
CrossRef
- Do AH, Wang PT, King CE, Abiri A, Nenadic Z. Brain-computer interface controlled functional electrical stimulation system for ankle movement. Journal of neuroengineering and rehabilitation. 2011;8:49.
CrossRef
- Hashimoto Y, Ota T, Mukaino M, Liu M, Ushiba J. Functional recovery from chronic writer’s cramp by brain-computer interface rehabilitation: a case report. BMC neuroscience. 2014;15:103.
CrossRef
- King CE, Wang PT, Mizuta M, Reinkensmeyer DJ, Do AH, Moromugi S, et al. Noninvasive brain-computer interface driven hand orthosis. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2011;2011:5786-9.
CrossRef
- Oken BS, Orhan U, Roark B, Erdogmus D, Fowler A, Mooney A, et al. Brain-computer interface with language model-electroencephalography fusion for locked-in syndrome. Neurorehabilitation and neural repair. 2014;28(4):387-94.
CrossRef
- Kandel ER, Schwartz JH, Jessell TM. Principles of neural science: McGraw-Hill New York; 2000.
- Bach R. Jonathan Livingston Seagull: Simon and Schuster; 1970.
- Yuan H, Liu T, Szarkowski R, Rios C, Ashe J, He B. Negative covariation between task-related responses in alpha/beta-band activity and BOLD in human sensorimotor cortex: an EEG and fMRI study of motor imagery and movements. NeuroImage. 2010;49(3):2596-606.
CrossRef
- Qin L, Ding L, He B. Motor imagery classification by means of source analysis for brain–computer interface applications. Journal of neural engineering. 2004;1(3):135.
CrossRef
- Schwarz D, Subramanian V, Zhuang K, Adamczyk C, editors. Educational Neurogaming: EEG-Controlled Videogames As Interactive Teaching Tools For Introductory Neuroscience. Tenth Artificial Intelligence and Interactive Digital Entertainment Conference; 2014.
- Bai O, Lin P, Vorbach S, Floeter MK, Hattori N, Hallett M. A high performance sensorimotor beta rhythm-based brain-computer interface associated with human natural motor behavior. Journal of neural engineering. 2008;5(1):24-35.
CrossRef
- Guger C, Schlogl A, Walterspacher D, Pfurtscheller G. Design of an EEG-based brain-computer interface (BCI) from standard components running in real-time under Windows. Biomedizinische Technik Biomedical engineering. 1999;44(1-2):12-6.
CrossRef
- Allison BZ, Pineda JA. ERPs evoked by different matrix sizes: implications for a brain computer interface (BCI) system. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2003;11(2):110-3.
CrossRef
- Ahn S, Ahn M, Cho H, Chan Jun S. Achieving a hybrid brain-computer interface with tactile selective attention and motor imagery. Journal of neural engineering. 2014;11(6):066004.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.